Researchers Find A New Way To Capture CO2


The team has created CO2 sorbents that are able to capture CO2 in larger numbers and at higher stability than previous attempts. Getting rid of the use of liquid sorbents, the new study works with amines on a porous solid, a much more stable method.
CO2 levels are higher than they have ever been and this is causing some serious problems for planet Earth. Climate change leads to weather becoming more unpredictable, with more instances of floods and droughts that dramatically disrupt many different living organisms. Minimizing CO2 levels is more important than it has ever been and scientists at the Tata Institute of Fundamental Research in Mumbai are leading new research that may offer a solution.
The one downside to older forms of capturing CO2 is that the amount of the gas that can be captured at any given moment is decreased due to the surface area and pore volume. Scientists from TIFR Mumbai have designed functionalized nanomaterials that offer a much larger capacity for amine loading along with very little decline in surface area.
Dr. Vivek Polshettiwar, lead scientists of the newest study says the fibrous nanosilica (also known as KCC-1) is a very good candidate to be used in a more functional design with a better amount of capture capacity as well as kinetics and recyclability. The surface area of KCC-1 is high due to its fibrous morphology. Other materials that have been studied for CO2 capture rely on their mesoporous channels.
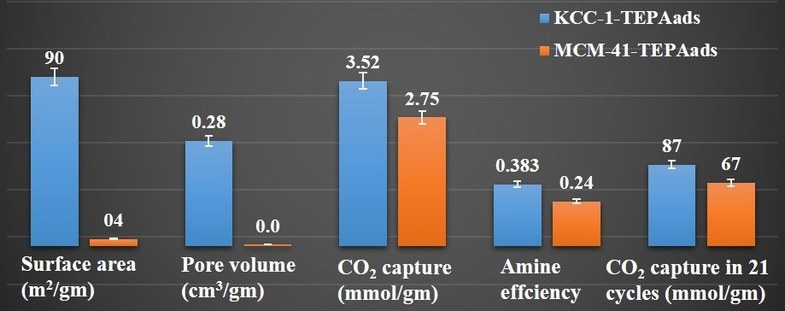
KCC-1 boasts a very high amine loading, very minor decrease in both surface area and functionalization, along with amine sites with improved accessibility. So far CO2 capture is a leading solution in the fight to reduce CO2 levels that show no signs of slowing down. Other materials, such as SBA-15 have received a lot of attention for their large pores which are able to hold a wide variety of amine molecules and bigger surface areas, but they do not have the high number of textural properties found in KCC-1.
The study was published in the Journal of Materials Chemistry A and compares the new and conventional solutions for lowering the amount of CO2 released into the atmosphere. Researchers continue to work on creating even better catalysts and sorbents with high sustainability.