Purifying Dirty Water to Make it Drinkable – New Nanomaterials Can Be a Game Changer


A new wonder material called graphene oxide may change drinking water as we now know it. This material creates a flexible, strong yet lightweight substance when it is incorporated into a foam made of nanocellulose. It is also efficient at conducting electricity and heat quickly. An engineering team at Washington University has discovered how this substance can change dirty water into a drinkable form. This could very well be a game-changer with worldwide implications.
Srikanth Singamaneni, a mechanical engineering and materials science associate professor at the School of Engineering & Applied Sciences says that countries like India that have ample sunlight may be able to use this material to evaporate dirty water and then collect water that is fresh and most of all drinkable.
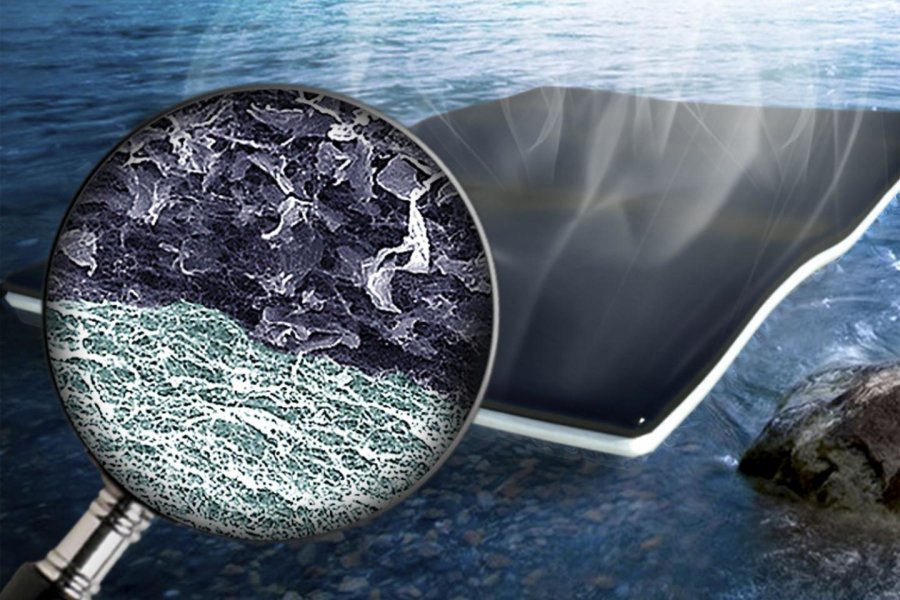
This is a brand-new approach combining graphene oxide and cellulose produced from bacteria to form a bioform that is bi-layered. According to Singamaneni, this is a simple process that involves moving the water using the nanoscale fiber network of cellulose. The water is moved to the surface so it will evaporate and this is done in one application while at the same time minimizing the amount of heat that is allowed down.
Singamaneni goes on to say that the material design is novel, producing a structure that is bi-layered with light-absorbing nanocellulose filled with graphene oxide sitting at the top while pristine nanocellulose rests at the bottom. When it is all suspended on water evaporation occurs because the water is pulled up to reach the surface. The graphene oxide converts light into heat but the amount of heat dissipation that is going to the underneath bulk water is minimized by the pristine nanocellulose. The heat should be confined only to the surface layer because that is where the evaporation is occurring.
Singamaneni also states that mass scale production of cellulose can be accomplished and graphene oxide is very inexpensive. They are both also very scalable so creating huge biofoam sheets is definitely a possibility. Along with its strength, biofoam is inexpensive to create and very lightweight. This makes it the perfect tool for desalination and water purification.
The biofoam bottom layer of cellulose performs as a sponge to draw water to the graphene oxide for fast evaporation. The fresh water is easy to collect from the top layer of the sheet.
The structure of the bi-layered biofoam is unique consisting of nanocellulose fibers that are formed by bacteria holding the flakes of graphene oxide. A pearl is formed by an oyster in much the same way.
According to the paper’s lead author and a Singamaneni lab graduate student, Qisheng Jiang, the flakes of graphene oxide are added while the cellulose bacteria is being cultured. He also states that the oxide gets embedded while the bacteria produce the cellulose. This creates an extremely strong interface. At one point in the process the graphene oxide medium is removed and a fresh medium is reintroduced, forming a new foam layer.
The foam material involves harvesting solar energy according to Pratim Biswas, chair of the Department of Energy, Environmental and Chemical Engineering and a Lucy and Stanley Lopata Professor. This makes it more effective with water cleanup and the synthesis process allows other materials that are nano-structured to enter the foam. With these added it will speed up the destruction rate of bacteria and various other contaminants to make the water drinkable. The novel structures produced also open the door for discovering other suitable applications.
Fully detailed study has been featured in the Advanced Materials journal.
Save