Water Disinfected in Minutes With Newly Developed Solar Gadget


To make water safe to drink, the germs in the water can be killed by either boiling the water, or by putting it out in the Sun in a plastic bottle, giving ultraviolet rays the opportunity to kill the microbes. In many parts of the world, clean water is not readily available and using tese methods is a way of life.
Boiling water uses fuel, which is often precious, while the UV method takes six to 48 hours as UV rays carry only 4 percent of the Sun’s total energy. This severely limits the volume of water that can be disinfected daily.
Researchers at Stanford University and the Department of Energy‘s SLAC National Accelerator Laboratory have manufactured a nanostructured device that can disinfect water much faster than the UV method. The device makes use of the visible part of the solar spectrum and is only about half the size of a postage stamp. As the visible part of the solar spectrum contains 50 percent of the Sun’s energy compared to the 4 percent in UV rays, it is reported that more than 99.999 percent of bacteria were killed in 20 minutes.

The experiments were reported in the journal of Nature Nanotechnology. The formation of various disinfecting chemicals, including hydrogen peroxide, was triggered by sunlight falling on the device. Once the bacteria had been killed, pure water was left behind due to the chemicals dissipating quickly.
Chong Liu is an investigator with SIMES (the Stanford Institute for Materials and Energy Sciences at SLAC) and a postdoctoral researcher in the laboratory of Yi Cui. As lead author, she explained that they simply dropped the device into the water and placed everything in sunlight. The Sun did all the work through the device that looks like a small rectangle of black glass.
When viewed with an electron microscope the surface of the device has many closely spaced lines, almost giving it the appearance of a fingerprint. The lines are called nanoflakes and are actually very thin films of molybdenum disulfide. These are stacked on edge on top of a rectangle of glass.
Molybdenum disulfide is normally used as an industrial lubricant. When it is however manufactured in layers just a few atoms thick, it takes on entirely different properties and becomes a photocatalyst. Many of its electrons leave their usual places when the film is hit by sunlight. The “holes” left by the electrons and the electrons themselves are then ready to participate in chemical reactions.
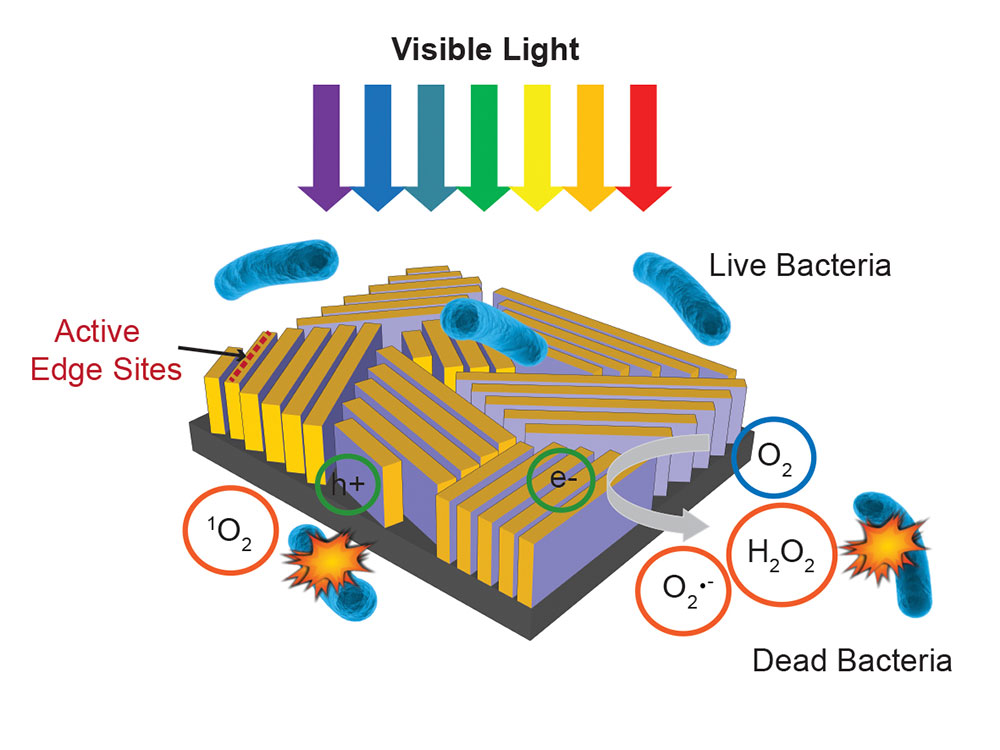
To be able to absorb the full range of visible sunlight, the thickness of the molybdenum disulfide walls has to be exactly right. Each minute wall is then topped with a thin layer of copper. The copper acts as a catalyst and, together with the walls, produce “reactive oxygen species” like hydrogen peroxide when hit by sunlight.
Molybdenum disulfide is easy to make and, more importantly, cheap. This is critical when making devices for extensive use in developing countries. It is also able to absorb a much wider range of solar wavelengths than old-style photocatalysts do.
Extensive further testing still needs to be done before commercial production of the device can begin. It has only been tested on three strains of bacteria mixed with water in the lab. The researchers believe that it will also kill other types of microbes, such as viruses, as well as other bacterial strains. It will however not be able to remove chemical pollutants from water and its effectiveness in the complex stews of contaminants found in third world countries remains to be proven.
Save