Super-Ions Set to Revolutionize the Solar Cell Industry
In 2009, hybrid perovskites based solar cells typically operated at 4% efficiency. Despite the steady improvements researchers have been able to make over the years to bring the efficiency up to the 20% it is today, the performance of perovskites in solar cells is still severely limited by a lack of stability. Another drawback is that hybrid perovskites contain toxic lead.
Research on using hybrid perovskites as the building blocks of a new generation of efficient optoelectronics devices and solar cells have been ongoing for a number of years. Scientists have been learning how to control different properties and the stability in these solar cell materials.
New design techniques have identified clusters of atoms that carry the same charge as the ions that they replace. Scientists can now customize these super-ion building blocks to improve both stability and other desired qualities. Simulations were used to develop the new design principles and that can lead to a brand new generation of product such as data storage, optoelectronics for lighting and solar cells. The new design principles will allow for manufacturing methods that are both environmentally friendly and simple.
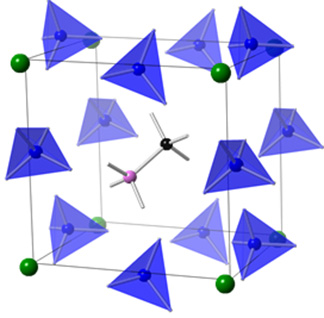
Virginia Commonwealth University has been leading a team of researchers in a comprehensive study to identify parameters and mechanism in over 40 materials with the aim of improving stability and control properties in lead-free hybrid perovskites. Simulations were performed to fill the knowledge gap for a series of lead-free hybrid perovskites where experimental data were not available. Ab initio molecular dynamics simulations, dynamic crystalline lattice calculations and density functional theory were amongst the many computational methods used. The material can be depicted as a super crystal composed of super-ions, both super-halogen and super-alkali ions.
By making changes in both the metal (germanium or tin) and halogen (chlorine, bromine, or iodine) in the super ions changed the ionic nature of the bonding and their ionic radii. The ionic nature of bonding to the electronic band gap was correlated to the electronic band gap and other photovoltaic-relevant properties.
This led to the team identifying two methods to increase the ionic nature: using a more metallic metal (tin compared to germanium) and using a smaller halogen to increase the radius ratio between super-ions. The scientists also identified how the materials degrade when they are exposed to moisture and proposed counter strategies based on this. This new understanding at an atomic-level could lead to the development of longer-lasting and more efficient solar cells.
Findings of this new discoveries have been published in-detail in the Journal of Materials Chemistry A.
Save